Scientific, ethical, and legal considerations for the
inclusion of pregnant people in clinical trials
Catherine A. Sewell, MD, MPH; Sarah M. Sheehan, MPA; Mira S. Gill, BA; Leslie Meltzer Henry, JD, PhD;
Christina Bucci-Rechtweg, MD; Cynthia Gyamfi-Bannerman, MD, MSc; Anne D. Lyerly, MD; Leslie C. Mc Kinney, PhD;
Kimberly P. Hatfield, PhD; Gerri R. Baer, MD; Leyla Sahin, MD; Christine P. Nguyen, MD
Introduction
The COVID-19 pandemic has under-
scored the ethical and health care de-
livery implications of the failure to enroll
pregnant people in clinical trials, and has
accentuated the already evident need for
efficacy and safety data to inform the use
of critical medical products during
pregnancy.
1
The data necessary to sup-
port the emergency use authorization of
COVID-19 prevention and treatment
measures for the adult population were
accrued with unprecedented speed,
outpacing the availability of clinical data
to supp ort timely enrollment of preg-
nant people in trials, and leading to
conflicting recommendations about
COVID-19 vaccine use in pregnancy.
2,3
Despite early studies indicating that
pregnant people are at higher risk of
developing complications owing to
COVID-19, and despite recommenda-
tions from the US Food and Drug
Administration (FDA) COVID-19
guidance
4,5
and other organizations
and experts for the inclusion of pregnant
Click Video under article title in Contents at
From the Division ofUrology, Obstetricsand Gynecology, United StatesFoodand Drug Administration, Silver Spring, MD (Dr Sewell); Duke-Margolis Center for
Health Policy, Washington, DC (Mses Sheehan and Gill); University of Maryland Carey School of Law and Johns Hopkins Berman Institute of Bioethics,
Baltimore, MD (Dr Henry); Novartis Pharmaceuticals Corporation, East Hanover, NJ (Dr Bucci-Rechtweg); Department of Obstetrics, Gynecology &
Reproductive Sciences, University of California San Diego School of Medicine, San Diego, CA (Dr Gyamfi-Bannerman); Department of Social Medicine and
Center for Bioethics, University of North Carolina at Chapel Hill, Chapel Hill, NC (Dr Lyerly); Division of Pharmacology Toxicology for Rare Diseases, Pediatrics,
Urology and Reproductive Medicine, United States Food and Drug Administration, Silver Spring, MD (Drs McKinney and Hatfield); Office of Pediatric
Therapeutics,United States Food and Drug Administration, Silver Spring, MD (Dr Baer);Division of Pediatricand Maternal Health, United States FoodandDrug
Administration, Silver Spring, MD (Dr Sahin); and Office of Rare Diseases, Pediatrics, Urology and Reproductive Medicine, United States Food and Drug
Administration, Silver Spring, MD (Dr Nguyen).
Received Dec. 10, 2021; revised June 17, 2022; accepted July 14, 2022.
The views expressed in this article are the personal views of the authors and may not be understood or quoted as being made on behalf of or reflecting the
position of their respective institutions, member organizations, employing companies, health agencies, or one of its committees or working parties.
C.G.B. participated in paid lectures for Medela on breastfeeding and COVID-19 and for Hologic on late preterm pregnancy. The remaining authors report
no conflict of interest.
This publication was supported by the U.S. Food and Drug Administration (FDA) of the U.S. Department of Health and Human Services (HHS) as part of a
financial assistance award U19FD006602, totaling $873,663, with 100% funded by FDA and HHS. The contents are those of the authors and do not
necessarily represent the official views of, nor an endorsement, by FDA, HHS, or the US Government.
0002-9378/$36.00 ª 2022 Elsevier Inc. All rights reserved. https://doi.org/10.1016/j.ajog.2022.07.037
Clinical trials to address the COVID-19 public health emergency have broadly excluded
pregnant people from participation, illustrating a long-standing trend of clinical trial exclusion
that has led to a clear knowledge gap and unmet need in the treatment and prevention of
medical conditions experienced during pregnancy and of pregnancy-related conditions. Drugs
(includes products such as drugs, biologics, biosimilars and vaccines) approved for a certain
medical condition in adults are also approved for use in pregnant adults with the same medical
condition, unless contraindicated for use in pregnancy. However, there are limited pregnancy-
specific data on risks and benefits of drugs in pregnant people, despite their approval for all
adults. The United States Food and Drug Administrationeapproved medical products are used
widely by pregnant people, 90% of whom take at least 1 medication during the course of their
pregnancy despite there being sparse data from clinical trials on these products in pregnancy.
This overall lack of clinical data precludes informed decision-making, causing clinicians and
pregnant patients to have to decide whether to pursue treatment without an adequate un-
derstanding of potential effects. Although some United States Food and Drug Administration
initiatives and other federal efforts have helped to promote the inclusion of pregnant people in
clinical research, broader collaboration and reforms are needed to address challenges related
to the design and conduct of trials that enroll pregnant people, and to forge a culture of
widespread inclusion of pregnant people in clinical research. This article summarizes the
scientific, ethical, and legal considerations governing research conducted during pregnancy, as
discussed during a recent subject matter expert convening held by the Duke-Margolis Center
for Health Policy and the United States Food and Drug Administration on this topic. This article
also recommends strategies for overcoming impediments to inclusion and trial conduct.
Key words: clinical trials, clinical trial conduct, fetal health, maternal health, research during
pregnancy, US Food and Drug Administration
DECEMBER 2022 American Journal of Obstetrics & Gynecology 805
Special Repor t ajog.org

people in clinical trials, COVID-19
medical product development did not
specifically focus on the particular con-
dition of pregnancy. The exclusion of
pregnant people in COVID-19 trials is
one example of their routine exclusion
from medical product trials more
broadly. In addition, there is little clinical
development of products for pregnancy-
related conditions. As a consequence of
these practices, both patients and their
providers often have sparse information,
if any, with which to assess the bene fits
and risks associated with medical prod-
uct use during pregnancy for both the
pregnant person and the developing
fetus. Limited or absent information can
lead to unsafe use of medical products or
refusal or reluctance to prescribe or
accept necessary treatment.
The COVID-19 medical product trials
illustrate a long-standing trend of
excluding pregnant people from clinical
trials for prescription and nonprescrip-
tion drug products and biologic prod-
ucts (including vaccines), which has led
to a clear knowledge gap and unmet need
for evidence to inform both pregnancy-
related conditions and medical condi-
tions existing in pregnancy. Approxi-
mately 90% of pregnant people take at
least 1 medication in pregnancy, and
70% of those who are pregnant take at
least 1 prescription medication.
6e8
Medical products that are approved for
adults are also approved for pregnant
populations because pregnant people are
adults, barring a contraindication for the
use of some products during pregnancy,
even if pregn ancy-specific data are lack-
ing. Dosing studies of medical products
for conditions that exist in pregnancy
often do not include pregnant people,
forcing clinicians to use their clinical
judgment to extrapolate appropriate
dosage for similar efficacy when pre-
scribing treatments in pregnancy,
potentially leading to ineffective treat-
ment or excess toxicity. This knowledge
gap, and the potential adverse impact on
patient care, underscores the importance
of planning for the collection of data
needed to support use in pregnancy at
the beginning of product development
programs. To address the knowledge
gap, it is critical that studies necessary to
support enrolling pregnant people in
clinical tr ials, including nonclinical
developmental and reproductive toxicity
studies and clinical pharmacology
studies, are conducted as early as
possible.
The overall lack of clinical data in
pregnancy presents important chal-
lenges that regulators and clinicians are
actively seeking to address. To guide and
manage the use of medical products in
pregnant populations, regulatory
decision-makers and clinicians must
often rely on safety data from nonclinical
studies and efficacy and safety studies in
the nonpregnant populati on. Even if
data in pregnant people are eventually
collected in the postapproval setting, this
accrual often occurs slowly; hence, the
evidence gap persists for an extended
period of time after product approval.
Furthermore, data collected in the
postapproval setting in pregnant people
are often subjected to inherent biases
and confounders that may not be
adequately mitigated.
To address this knowledge gap and
encourage timely evidence generation
for pregnant populations, federal
agencies, patient organizations, and
collaborative publiceprivate partner-
ships have actively raised awareness of
key issues and are taking steps to boost
inclusion of pregnant people in clinical
trials (Table).
Although these initiatives have helped
promote the inclusion of pregnant peo-
ple in clinical research, broader collab-
oration and reforms are needed to
address challenges related to the design
and conduct of trials that enroll pregnant
people and to forge a culture of wide-
spread inclusion of pregnant people in
research.
Accordingly, the Duke-Margolis
Center for Health Policy, under a coop-
erative agreement with the FDA,
convened a public meeting in February
2021 to discuss the scientific and ethical
considerations for including pregnant
people in clinical trials. Meeting partic-
ipants represented a wide variety of
stakeholder categories including
academia, industry, governmental
agencies, and patient advocacy groups.
Below, we summarize key input from
meeting participants about the scientific,
ethical, and legal considerations gov-
erning research for pregnancy, and pro-
vide their recommendations on
approaches to overcome impediments to
including and conducting trials in preg-
nant people.
Scientific considerations
Nonclinical and clinical data are essential
components of drug development, and
both are critical for regulatory and clin-
ical decision-making for all patient
populations. The scientific consider-
ations and associated challenges dis-
cussed during the me eting fall into the
following categories:
1. Nonclinical studi es to support the
conduct of clinical trials in pregnant
people
2. Clinical data collection to support
regulatory decision-making and
evidence-based care delivery
Nonclinical studies to support trial
conduct
Nonclinical studies provide key safety
information that informs clinical trial
eligibility criteria, dosing decisions, and
drug labeling. Generally, nonclinical
studies to support enrolling pregnant
people in clinical trials include nonclin-
ical safety assessments from reproduc-
tive and developmental toxicity studies
in animals. At the meeting, representa-
tives from the FDA described the char-
acteristics of standard nonclinical
studies and their utility and limitations.
Detailed information regarding general
and specific nonclinical study conduct
and design can be found in a multitude
of published guidances by FDA, and in
conjunction with worldwide regulatory
agencies through the International
Council for Harmonisation of Technical
Requirements for Pharmaceuticals for
Human Use (ICH). Two pertinent re-
sources for this topic are ICH M3 (R2)
Nonclinical Safety Studies for the
Conduct of Human Clinical Trials and
Marketing Authorization for Pharma-
ceuticals, and ICH S5 (R3) Detection of
Reproductive and Developmental
Toxicity for Human Pharmaceuticals.
9,10
Special Report ajog.org
806 American Journal of Obstetrics & Gynecology DECEMBER 2022
Meeting participants primarily dis-
cussed the characteristics and re-
quirements of 2 types of noncli nical
studies: reproductive toxicity and devel-
opmental toxicity studies. Reproductive
toxicity studies assess how drugs may
affect the reproductive competence of
sexually mature males and females.
Developmental toxicity studies assess
possible adverse effects from drug
exposure from preconception, prenatal,
or postnatal exposure on the developing
fetus up to sexual maturity. These
nonclinical studies use animal models
(typically rats and rabbits) that allow
safety assessment across gestational
stages, can be completed efficiently, and
are intended to inform safe use of med-
ical products throughout pregnancy.
These nonclinical animal models are
well established and accepted worldwide
for drug registration, have significant
background control data, have targeted
treatment periods with specific end-
points, and are intended to interrogate
high doses of drug molecules.
In general, pregnancy-related info r-
mation in the drug label to inform pre-
scribing decisions in the pregnant
person consists solely of evidence from
nonclinical studies at the time of dru g
approval. Although nonclinical studies
provide essential information, they also
have limitations. First, although rats and
rabbits serve as effective animal models,
the inherent species differences between
these animals and humans can lead to
some uncertainties when extrapolating
from animals to humans. For example,
some studies in animals may produce
safety signals at high doses that would
not appear in humans because of species
and related dosage differences, and thus
may provide some assurance for human
safety. Conversely, animal studies may
not capture harms that may occur in
human pregnancies. In addition, there
are study design limitations that affect
the translational utility of available ani-
mal models. For example, nonclinical
reproductive and developmental studies
are designed to capture only gross
morphologic and easily observable
functional effects of a drug. Thus, stan-
dard study designs do not include as-
sessments of drug effects on higher-
order learning and memory, immune
system development, endocrine system
functioning following puberty, or ani-
mal fetal exposure levels; although
specially designed studies can capture
these data if there is a known concern
that warrants these special studies.
Although nonclinical studies have
limitations, they are a vital component to
informing clinical research in pregnant
populations and must be completed
before progression to later stages of
clinical trials that enroll pregnant people.
Thus, the timing of reproductive and
developmental studies in the drug
development program is critical when
planning for trials enrolling pregnant
people. Although it would seem ideal to
perform these nonclinical studies early
to inform risk, doing so befo re knowing
the doses to be investigated in a
nonpregnant population in a clinical
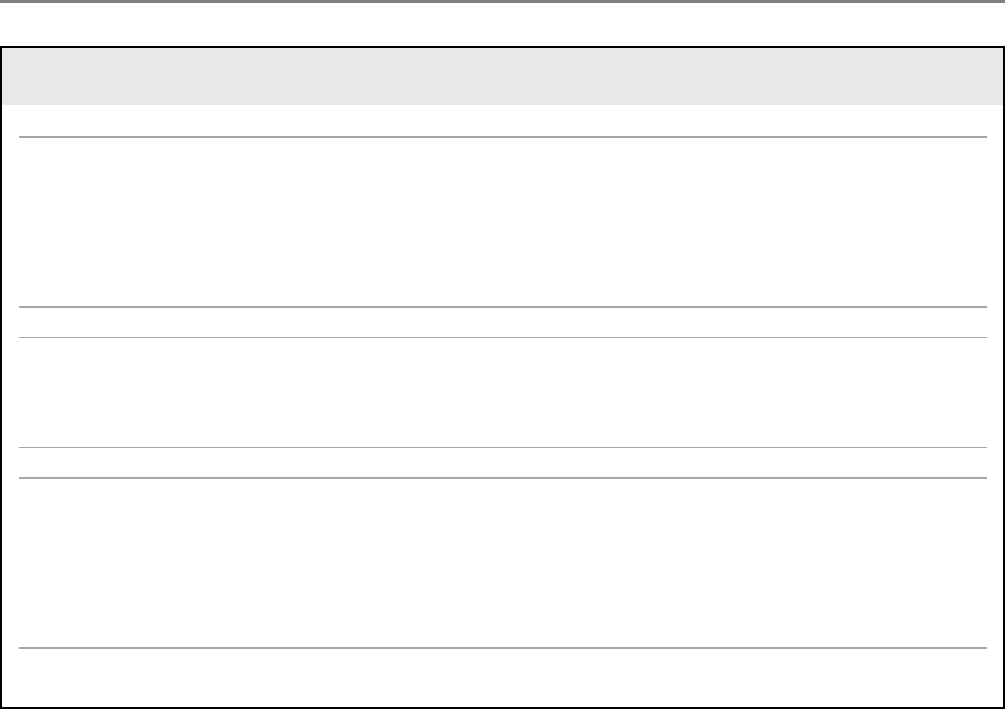
TABLE
Federal efforts to advance therapeutic research in pregnancy
FDA initiatives
FDA Office of Women’s Health
18
:
Advises the FDA Commissioner on
topics related to women’s health
Provides funding for research related
to women’s health
Maintains a pregnancy registry webpage,
supports workshops, and develops educa-
tional resources for pregnant people
FDA Perinatal Health Center of Excellence
19
:
Collaborates with other
FDA centers and external organizations to
support research to advance regulatory
science for perinatal populations
FDA draft guidance:
Pregnant Women: Scientific and Ethical
Considerations for Inclusion in Clinical Trials
Postapproval Pregnancy Safety Studies
National Institutes of Health initiatives
PregSource
20
:
Collects information from pregnant
people about pregnancy and overall
maternal health
Maternal and Pediatric Precision in
Therapeutics (MPRINT) Hub
21
:
Collects tools and
data to further maternal and pediatric
therapeutic development
Pregnancy and HIV/AIDS: Seeking Equitable
Study (PHASES)
22
:
Developing guidance for conduct of clinical
trials for HIV in pregnancy
PRGLAC
PRGLAC Report to the HHS Secretary and
Congress, September 2018
7
:
Describes knowledge gaps and ethical
considerations related to research in
pregnant and lactating people
Includes the Task Force’s 15 recommenda-
tions to improve therapeutic development for
pregnant and lactating people
PRGLAC Report Implementation Plan to HHS
Secretary, August 2020
23
:
Outlines implementation plan for the Task
Force’s 15 recommendations
FDA, Food and Drug Administration; HHS, United States Department of Health and Human Services; PRGLAC, Task Force on Research Specific to Pregnant Women and Lactating Women.
Sewell. Inclusion of pregnant people in clinical trials. Am J Obstet Gynecol 2022.
ajog.org Specia l Report
DECEMBER 2022 American Journal of Obstetrics & Gynecology 807

trial can increase overall cost, prolong
data collection timelines for the general
drug development program, and may
delay therapeutic availability to the
general population. For most drugs
intended for the general population,
fertility and early embryonic develop-
ment (FEED) and embryofetal develop-
ment (EFD) studies are not conducted
until after or concurrently with Phase 2
studies, whereas pre- and postnatal
development (PPND) studies are often
concurrent or prior to Phase 3. For drugs
expected to be used by people who may
need treatment in pregnancy, con-
ducting these nonclinical studies early in
drug development to specifically ident ify
any pregnancy-related risks would allow
earlier inclusion of pregnant people in
clinical trials and facilitate the timely
collection of pregnancy-specific human
data before product approval.
Clinical data collection for decision-
making
Physiological considerations
Clinical research during pregnancy is
critical for supporting safe and effec-
tive drug use because physiological
changes during pregnancy can affect
the pharmacokinetics (PK) of a drug
and thus the dosage needed to reach
the intended therapeutic effect
compared with nonpregnant pop-
ulations. Such physiological changes
include: doubling of blood volume,
fluctuations in levels of circulating
binding proteins, slower gastrointes-
tinal transit time, and alterations in
me tabolism a nd excretion. Each of
these changes can var y over the dura-
tion of the pregnancy, thus it is
important to study therapeutic effect
and dosing throughout gestation.
Numerous patient characteristics and
medical conditions may coexist with
pregnancy, such as diabetes mellitus,
hypertension, chronic kidney disease,
and liver disease that can affect drug
metabolism, further underscoring the
need for robust PK studies in pregnant
people to guide safe and effective dosing.
In addition, fetal and placental develop-
ment can affect dispo sition of thera-
peutics during pregnancy because
different gestational stages are associated
with different susceptibilities and fetal
and placental physiological changes.
Absent knowledge of PK in pregnancy,
the appropriate modifications in dosing
to match physiological changes are un-
known and may result in either under-
dosing and inadequate treatment, or
overdosing leading to toxicities.
Trial design consi derations
Clinical research considerations also
include those related to trial design and
conduct. Clinical trial design and
conduct are influenced by factors such as
the drug class, study objectives, disease
area, and therapeutic context. Thera-
peutic context depends on various fac-
tors includ ing the nature of the disease,
whether there is an unmet medical need,
availability of treatments, and the po-
tential benefits and risks of a medical
product.
The objectives of clinical trials during
pregnancy can be divided into 2 broad
categories:
1. To support the development of ther-
apeutics for pregnancy-related con-
ditions (eg, preterm birth,
preeclampsia, intrahepatic chole-
stasis of pregnancy)
2. To support the development of ther-
apeutics for medical conditions that
coexist with pregnancy (eg, hyper-
tension, diabetes mellitus, COVID-
19)
In addition to clinical trial design,
there was extensive discussion at the
meeting about trial conduct and clinical
data collection considerations to allow
timely accrual of evidence for the use of
medical products in pregnancy. For
example, participants discussed a pro-
posed framework for conducting clinical
trials in pregnant people earlier in drug
development. Participants emphasized
that it was both possible and preferable
to begin evaluation in pregna nt people
during Phase 3 or earlier, as opposed to
conducting it solely in the postapproval
setting, with appropriate consideration
given to therapeutic context.
Participants noted there were major
operational barriers to pursuing some of
these approaches in both private- and
public-sector research. These barriers
are discussed in the legal considerations
section.
Ethical considerations
Meeting participants from all stake-
holder categories agreed that reticence to
include pregnant people in trials pre-
vents collection of data that inform as-
sessments of safety, efficacy, and
therapeutic dosage, thereby precluding
adequate information for informed
decision-making in pregnancy. Howev-
er, several participants noted that bar-
riers to enrolling pregnant people in
clinical trials persist largely because of
ethical concerns and outdated or mis-
informed ideas about clinical research.
A meeting participant specializing in
bioethics stated that clinical research in
pregnant people has been guided by a
protectionist ethic, which has ultimately
had harmful consequences. The protec-
tionist ethic has limited the autonomy of
pregnant persons, led to their exclusion
from research, and exposed them and
their children to harms of constrained
evidence. The protectionist ethic mani-
fests, for example, in a regulation guid-
ing clinical trials in pregnancy that
requires paternal consent, in addition to
maternal consent, when the prospect of
direct benefit applies only to the fetus.
This requirement fails to acknowledge
that the interests of a pregnant person
and their fetus are intertwined strands in
contrast to research conducted in pedi-
atric settings, where the consent of one
parent is sufficient to authorize research
with a prospect of direct benefittoa
child. In addition, participants under-
scored the importance of considering
altruism given that pregnant people may
choose to participate in research not only
when there is a direct benefit to study
participan ts but also when the research
could benefit other current and future
pregnant people.
Furthermore, researchers discussed
the ethical implications of requiring
contraception in certain trials where it
would not be needed or medically
acceptable to do so. Participants high-
lighted that requiring contraception in
cases where there is no prospect of
pregnancy (eg , a trial participant in a
Special Report ajog.org
808 American Journal of Obstetrics & Gynecology DECEMBER 2022

same-sex relationship) raises ethic al
concerns by imposing unnecessary re-
quirements or preventing participation
in a study.
Ultimately, the meeting participant
emphasized the ethical principle of
protecting pregnant people, not from
research, but through research. In pre-
venting research in pregnant people, the
protectionist ethic does not in fact
eliminate or mitigate potential harm
from medical products. Rather, the po-
tential for harm remains unknown in a
clinical setting where prescribers and
patients must make decisions about
treatment, potentially exposing a larger
number of individuals to adverse events
than would occur in a trial setting.
Including pregnant people in clinical
trials provides the opportunity to assess
potential risks in a controlled setting, so
that risk mitigation strategies can be
identified to inform clinical care.
11
Finally, efforts to “protect” pregnant
people from research, which inevitably
lead to insufficient data, have led to the
reluctance to prescribe or accept neces-
sary treatment, putting pregnant people
in harm’s way from absent or under-
treatment.
Another meeti ng participant special-
izing in bioethics discussed 2 over-
arching ethical questions associated with
trial conduct and developing an ethical
framework. The first question was:
“When is it ethically permissible to allow
enrollment of pregnant people into
clinical research?” Participants noted
that researchers should first assess
whether there is preliminary evidence
during a drug development program
indicating potential safety signals. In
addition, researchers should decide
whether the development program in-
volves acceptable research-related risk.
This includes assessing the benefiterisk
ratio rather than just the poten tial risk.
Thus, it generally follows that the more
potential benefit offered through
participation in a given trial, the greater
the risk that might be acceptable to the
research participant.
The second question highlighted was:
“When do we have an ethical re-
sponsibility to enroll pregnant people in
a trial (eg, if it is permissible to enroll, do
we have a responsibility to enroll)?”
Participants noted that researchers
should consider the deg ree to which
obtaining adequate evidence for the use
of medication in pregnancy, or access to
prospect of benefit from trial participa-
tion raises concerns of justice, and if so,
whether exclusion of pregnant people is
appropriate.
Meeting participants emphasized that
the ethical framework governing
enrolling pregnant people in clinical
trials should be based on protecting
pregnant people through inclusion in
research. For example, participants
noted that institutional review boards
(IRBs), funder s, and other stakeholders
can elevate the ethical responsibility to
include pregnant people in research by
requesting justification for their exclu-
sion when pregnant people are excluded
from research. The development of a
framework or common criteria consid-
ered adequate justification for exclusion
could facilitate such efforts.
Legal considerations
In addition to ethical considerations,
participants discussed how legal con-
siderations, such as perceptions of lia-
bility, dissuade industry sponsors and
research institutions from enrolling
pregnant people in clinical trials. One
legal exper t highlighted 4 key legal con-
siderations that influence stakeholder
decision-making about whether to
include pregnant people in clinical trials.
First, the participant noted, there is a
myth that including pregnant people in
research is legally impermissible.
Although FDA guidance and federal
regulations set forth criteria for ethically
and legally conducting such
research,
12,13
some members of the
research enterprise mistakenly believe
that the law precludes research during
pregnancy.
14
That misperception of the
law can lead to decisions—by industry
sponsors, academic institutions, clinical
investigators, and others along the
research pathway—that exclude preg-
nant peop le from research.
15
Second, this myth is amplified by a
combination of absent and ambiguous
regulations. Current federal regulations
governing research during pregnancy
(commonly referred to as “Subpart B”
16
)
neither require the inclusion, nor
penalize the unjustified exclusion, of
pregnant people in research. In addition,
Subpart B contains ambiguities, such as
the concept of “minimal risk,” that are
open to w ide interpretation. In the face
of regulatory ambiguity, and without a
clear directive to include pregnant peo-
ple in research, stakeholders can apply
conservative regulatory interpretations
that limit the inclusion of pregnant
people in clinical trials.
Third, inclusion of pregnant people
in a clinical trial may increase trial and
overhead expenses (eg, expenses
related to liability cove rage). Inclusion
may also lead to slower trial recruit-
ment if a minimum sample of preg-
nant people is required, leading to
delays in trial completion and drug
approval. Exclusion, by contrast, not
only allows researchers to avoid those
costs and delays, but also mitigates
risks for industry by eliminating
premarket liability and shifting p ost-
market liability to prescribers and pa-
tients, who ultimately decide whether
to use medications w ithout pregnancy-
sp eci ficdata.
Fourth, although liability is
frequently cited as a reason that deci-
sion makers exclude pregnant people
from research, the legal expert high-
lighted that most stakeholders’ fears of
liability for harms to pregnant subjects
and their fetuses and offspr ing exceed
evidence of actual liability. Although
premarket testing is not risk-free, lia-
bility is limited to the size of the
research population and may be miti-
gated by obtaining fully informed con-
sent from each participant in research.
By contrast, legal risk might increase
substantially after a drug enters the
market if, for example, adverse events
occur in a patient population that was
excluded from clinical research.
Finally, the legal expert and other
panelists highlighted several key legal
strategies to advance inclusion of preg-
nant people in clinical trials. First, it is
imperative to determine the degree of,
and mitigate, liability stemming from
premarket clinical trials. Risk mitigation
strategies, such as implementing
ajog.org Specia l Report
DECEMBER 2022 American Journal of Obstetrics & Gynecology 809

programs that provide compensation for
research-related injuries, can dampen
disincentives to including pregnant
people in research.
17
Second, incentiv-
izing research during pregn ancy will also
promote inclusion of pregnant people in
clinical trials. For example, public
funding opportunities or accelerated
drug review could serve to incentivize
clinical research in pregnant pop-
ulations. All participants agreed that
concrete liability reforms and targeted
educational initiatives are necessary for
encouraging clinical research in preg-
nant people. Furthermore, to accom-
plish the goals listed above, clinical
research in pregnancy must be seen as a
critical public health issue, and legal ex-
perts, from both within and outside of
the research enterprise, should be
engaged to develop workable strategies.
Next steps
To ensure appropriate enrollment of
pregnant people in clinical trials, and
address evidence gaps that stem from lack
of enrollment, there must be a shift toward
creating a culture of inclusion of pregnant
people in clinical trials rather than
routinely excluding them without
adequate justification. As mentioned
above, the clinical research community can
aid in cultivating this, in part, by framing
the evidence gap as a critical public health
concern. Doing so would raise awar eness
for this crucial issue and illustrate the need
for collaborati on among various stake-
holders to affect widespread change.
In the absence of legislative authorities
for FDA, the research community and
government stakeholders can also begin
holding investigators accountable for
including pregnant people in research by
encouraging inclusion or requesting a
justification for their exclusion before a
trial receives IRB authorization. Support
for these measures is building. These
steps can be taken without new regula-
tions and guidelines because the existing
regulatory oversight infrastructure is
sufficient to support inclusion of preg-
nant people in clinical research.
Although creating a culture of inclu-
sion will take time, stakeholders can take
steps now to identify and address
priority evidence gaps and prioritize re-
sources on the basis of unmet need. For
example, researchers can begin by
studying medications that are already
being used in pregnan t people but for
which there are no objective data derived
from clinical research to support the
medication use in pregnant people. In
addition, the clinical research commu-
nity can encourage stakeholders to
develop criteria (eg, frequency of use,
seriousness of unmet medical need) for
identifying priority medications for
study in pregnancy and devote resources
accordingly. Researchers can leverage
existing data and trial infrastructure to
obtain evidence to facilitate regulatory
and clinical decision-making for drugs
already being used by pregnant people in
the postmarket setting.
Meeting participants from industry,
federal agencies, and academic
research institutions alike described
how existing data such as electronic
health records and registry data could
be leveraged to support evidence gen-
eration. In addition, data collection
through tr ial networks that have access
to more clinical sites and patients
couldsupportincreasedenrollment
and maximize data utility, whereas
alternative data sources can be used to
supplement clinical data collection for
regulatory submissi ons and clinical
decision-making.
Finally, increased collaboration
among stakeholders (federal agencies,
industry, academia, and patients and
patient advocates) involved in each
phase of drug development can sup-
port progress in a variety of ways. First,
stakeholder coop eration can encourage
liability reform and education. Second,
increasing collaboration can aid in the
implementation of innovative trial ap-
proaches, such as the development of
master protocols, to reduce time- and
cost-relat ed burdens and encourage
earlier enrollment of pregnant people
in drug development. Third, increasing
collaboration and communication with
the FDA to suppor t timely collection of
nonclinical data and discuss study
design can facilitate earlier enrollment
of pregnant people in clinical trials.
Finally, increasing both public and
private funding to suppor t nonclinical
and clinical data collection can fur ther
incentivize and support clinical
research in pregnancy.
-
ACKNOWLEDGMENTS
We would like to thank all the speakers and
panelists who participated in the Duke-Margolis
Center for Health Policy and US Food and Drug
Administration meeting entitled “Scientific and
Ethical Considerations for the Inclusion of
Pregnant Women in Clinical Trials” in
February, 2021.
REFERENCES
1. Modi N, Ayres-de-Campos D, Bancalari E,
et al. Equity in coronavirus disease 2019 vaccine
development and deployment. Am J Obstet
Gynecol 2021;224:423–7.
2. American College of Obstetricians and Gy-
necologists. Vaccination site recommendations
for pregnant individuals. 2021. Available at:
https://www.acog.org/covid-19/vaccination-
site-recommendations-pregnant-individuals.
Accessed October 25, 2021.
3. World Health Organization. The Pfizer Bio-
NTech (BNT162b2) COVID-19 vaccine: What
you need to know. 2021. Available at: https://
www.who.int/news-room/feature-stories/
detail/who-can-take-the-pfizer-biontech-
covid-19– vaccine. Accessed October 25,
2021.
4. US Food and Drug Administration. Covid-19:
developing drugs and biological products for
treatment or prevention guidance for industry.
2021. Available at: https://www.fda.gov/media/
137926/download. Accessed October 25,
2021.
5. US Food and Drug Administration. Develop-
ment of monoclonal antibody products targeting
SARS-CoV-2, including addressing the impact
of emerging variants, during the covid-19 public
health emergency guidance for industry. 2021.
Available at: https://www.fda.gov/media/
146173/download. Accessed October 25,
2021.
6. Lyerly AD, Little MO, Faden R. The second
wave: toward responsible inclusion of pregnant
women in research. Int J Fem Approaches Bio-
eth 2008;1:5–22.
7. Eunice Kennedy Shriver National Institute of
Child Health and Human Development. Task
Force on Research Specific to Pregnant Women
Report to Secretary, Health and Human Ser-
vices Congress. 2018. Available at: https://
www.nichd.nih.gov/sites/default/files/2018-09/
PRGLAC_Report.pdf. Accessed October 25,
2021.
8. Mitchell AA, Gilboa SM, Werler MM,
Kelley KE, Louik C, Hernández-Díaz S. Medica-
tion use during pregnancy, with particular focus
Special Report ajog.org
810 American Journal of Obstetrics & Gynecology DECEMBER 2022

on prescription drugs: 1976-2008. Am J Obstet
Gynecol 2011;205:51.e1–8.
9. US Food and Drug Administration and Inter-
national Council for Harmonization. M3(R2)
Nonclinical safety studies for the conduct of
human clinical trials and marketing authorization
for pharmaceuticals. 2010. Available at: https://
www.fda.gov/media/71542/download. Accessed
June 6, 2022.
10. U.S. Food and Drug Administration and In-
ternational Council for Harmonization. S5(R3)
Detection of reproductive and developmental
toxicity for human pharmaceuticals guidance for
industry. 2021. Available at: https://www.fda.
gov/media/148475/download. Accessed June
6, 2022.
11. The American College of Obstetricians and
Gynecologists. Ethical considerations for
including women as research participants.
2015. Available at: https://www.acog.org/
clinical/clinical-guidance/committee-opinion/
articles/2015/11/ethical-considerations-for-
including-women-as-research-participants.
Accessed June 16, 2022.
12. Food and Drug Administration. Draft guid-
ance, pregnant women: scientific and ethical
considerations for inclusion in clinical trials.
Federal Register. 2018. Available at: https://
www.govinfo.gov/content/pkg/FR-2018-
04-09/pdf/2 018-07151.pdf. Accessed
August 4, 2021.
13. Department of Health and Human Services.
Additional protections for pregnant women,
human fetuses and neonates involved in
research. 2001. Available at: https://www.
govinfo.gov/content/pkg/FR-2001-11-13/pdf/
01-28440.pdf. Accessed August 4, 2021.
14. Shields KE. Pregnancy and the Pharma-
ceutical Industry: The Movement Towards
Evidence-Based Pharmacotherapy for
Pregnant Women. London: Academic Press;
2019.
15. Mastroianni AC, Henry LM, Robinson D,
et al. Research with pregnant women: new in-
sights on legal decision-making. Hastings Cent
Rep 2017;47:38–45.
16. Office for Human Research Protections.
Additional protections for pregnant women,
human fetuses and neonates involved in
research. 2019. Available at: https://www.hhs.
gov/ohrp/regulations-and-policy/reg ulations/
45-cfr-46/common-rule-subpart-b/index.
html. Accessed October 25, 2021.
17. Henry LM, Larkin ME, Pike ER. Just
compensation: a no-fault proposal for research-
related injuries. J Law Biosci 2015;2:645–68.
18. US Food and Drug Administration. Office of
Women’s Health. 2019. Available at: https://
www.fda.gov/about-fda/office-commissioner/
office-womens-health. Accessed July 16, 2021.
19. US Food and Drug Administration. National
Center for Toxicological Research. Perinatal and
maternal research. 2021. Available at: https://
www.fda.gov/ab out-fda/nctr -researc h-focus-
areas/perinatal-and-maternal-research.
Accessed July 16, 2021.
20. National Institutes of Health. What is Pre-
gSource. 2021. Available at: https://pregsource.
nih.gov/. Accessed July 16, 2021.
21. Eunice Kennedy Shriver National Institute of
Child Health and Human Development. Maternal
and Pediatric Precision in Therapeutics
(MPRINT) Hub. 2022. Available at: https://www.
nichd.nih.gov/about/org/der/branches/opptb/
mprint#more. Accessed July 16, 2021.
22. US National Institutes of Health. PHASES:
pregnancy & HIV/AIDS: seeking equitable
study. 2016. Available at: http://www.
hivpregnancyethics.org/about-1. Accessed
October 25, 2021.
23. Eunice Kennedy Shriver National Institute
of Child Health and Human Development.
Task force on research specific to pregnant
women and lactating women. Report Imple-
mentation Plan. 2020. Available at: https://
www.nichd.nih.gov/about/advisory/PRGLAC.
Accessed July 16, 2021.
ajog.org Specia l Report
DECEMBER 2022 American Journal of Obstetrics & Gynecology 811
